
The barium cation is written Ba 2+, not Ba +2. Note the convention of first writing the number and then the sign on a multiply charged ion. Figure 3.3 “Predicting Ionic Charges” shows how the charge on many ions can be predicted by the location of an element on the periodic table. On the other side of the periodic table, the next-to-last column, the halogens, form ions having a 1− charge. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. For example, all ions made from alkali metals, the first column on the periodic table, have a 1+ charge. Thus, the periodic table becomes a tool for remembering the charges on many ions. In many cases, elements that belong to the same group (vertical column) on the periodic table form ions with the same charge because they have the same number of valence electrons. In macroscopic samples of sodium chloride, there are billions and billions of sodium and chloride ions, although there is always the same number of cations and anions. The number of electrons lost by the sodium atom (one) equals the number of electrons gained by the chlorine atom (one), so the compound is electrically neutral. Notice that there are no leftover electrons. Thus, for sodium that has eleven electrons, the first energy level, which has the s sub orbit carries two electrons 1 s 2 1s2 1s2. The resulting combination is the compound sodium chloride. With two oppositely charged ions, there is an electrostatic attraction between them because opposite charges attract. On the right, the chloride ion has 18 electrons and has a 1− charge. Most nonmetals become anions when they make ionic compounds.įigure 3.2 The Formation of a Chlorine Ion. On the left, the chlorine atom has 17 electrons.
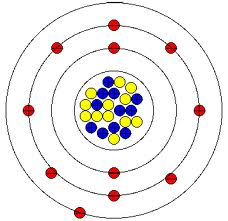
Negatively charged ions are called anions. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. Most metals become cations when they make ionic compounds.

Positively charged ions are called cations. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons in the nucleus. These patterns do not fill the outermost shell or satisfy the. In cases where an atom has three or fewer valence electrons, the atom may lose those valence electrons quite easily until what remains is a lower shell that contains an octet. In contrast, chlorine has only seven electrons in its outermost shell, while sodium has just one. Atoms are electrically neutral because the number of protons, which carry a 1+ charge, in the nucleus of an atom is equal to the number of electrons, which carry a 1- charge, in the atom. Image credit: Wikipedia Commons, public domain. Some atoms have only a few electrons in their outer shell, while some atoms lack only one or two electrons to have an octet. Sodium chloride is an ionic compound made up of sodium ions and chloride ions in a crystal lattice. Most atoms do not have eight electrons in their valence electron shell.


 0 kommentar(er)
0 kommentar(er)
